June 2024
I. Introduction
With a view to standardizing CMC studies and changes of biological products in clinical trials, fulfilling general requirements for samples used in different stages of clinical trials, and accelerating the progress of clinical trials and marketing of biological products and facilitating full-lifecycle management of biological products, this technical guideline is developed in accordance with regulations and requirements in the current Drug Administration Law of the People’s Republic of China, Vaccine Administration Law of the People’s Republic of China, Provisions for Drug Registration and Provisions for Post-approval Changes of Drugs (Interim).
CMC studies of biological products during clinical trials are characterized as progressive and staged. Following the development rule of biological products, advancing CMC studies and changes and ensuring sufficient CMC study data available to support the clinical trials are an important goal for the development of biological products during clinical trials and are also the basis for promoting clinical trials and submission of the BLA.
This guideline is in line with CMC technical requirements on INDs application for biological products with the satisfaction of the CMC requirements for BLAs as the goal. It is intended to, on the basis of reference and learning from the relevant technical guidelines at home and abroad, from a technical perspective, narrate the approaches to continuation of CMC studies of biological products during clinical trials and phased requirements on study content for biological products. In addition, it guides and standardizes the application for CMC changes to biological products during clinical trials by exemplifying CMC change items during clinical trials that may increase the safety risk of biological products.
This guideline is applicable to the biological products that have received implicit approval for clinical trials within the mainland China, including preventive biological products and therapeutic biological products, involves the CMC changes and/or updates in the whole “stages of clinical trials” from approval of IND application by implication to the submission of the BLA applications and covers the studies and changes for raw materials used in manufacturing, manufacturing processes, quality, stability and container closure systems etc. For the studies and changes for gene therapy biological products and cell therapy biological products during clinical trials, this guideline can also be referenced and studies of product specific changes should be conducted with the corresponding technical guidelines taken into account. This guideline is not applicable to in vitro diagnostic reagents used for blood screening.
Biological products are complex and diverse. This guideline, the general requirements for CMC studies and changes of biological products during clinical trials, only reflect the current opinion and understanding. For CMC studies and changes of biological products during clinical trials, the sufficient evaluation and studies could be carried out by referring to this guideline and taking into account of the characteristics of products, promoting the marketing and development progress of biological products.
II. General Principles
(I) Basic considerations 1. Scientific planning
To ensure the orderly development of biological products and minimize the impact caused by unexpected changes, it is necessary to scientifically plan CMC studies and manage change of biological products during clinical trials. Due to limited study data, R&D experience and prior knowledge (such as platform technology) are mainly based for the plan in early-stage clinical trials. With the acquisition of clinical data and the accumulation of CMC studies data, such plan should also be improved accordingly.
Platform technology could facilitate rapid development of innovative products and provides supporting data for risk assessment of changes during product development. Taking into account of the phased consideration of clinical changes and differences of post-marketing changes, the platform technology herein refers to one or a series of technologies used by the manufacturer to develop and/or produce similar products, and the previous products using this technology have been marketed or have been under clinical study. If, in the subsequent product development, such elements as product technology skeleton (may including nucleic acid carrier, virus carrier, protein skeleton, etc.), manufacturing processes, critical quality attributes and GMP implementation are basically unchanged, it can be regarded as a platform. Prior experience and knowledge gained with the platform, manufacturing data (on manufacturing, control and stability), and method validation can be used as supporting data to more rapidly develop new products that meet the boundaries of the platform and assess CMC changes during the development of new products. In case of use of platform technology for risk assessment, sufficient supporting basis shall be provided, such as comparative analysis of specific CMC study data, analysis related to mechanism of action, and previous clinical study progress.
2. Phased considerations
The CMC studies of biological products during clinical trials are gradually improved to be in line with the drug development rule. CMC changes during clinical trials are essentially a process of continuous enrichment, improvement and revision of CMC study information. Different from the CMC studies and changes prior to initial IND application studies and after marketing, CMC studies and changes of biological products during clinical trials are aimed at making risks and benefits balance, making early CMC study data supportive of the conduct of subsequent clinical trials and providing sufficient basis for the final marketing approval of biological products without additionally increasing the risk to the safety in clinical trial subjects.
During early-stage clinical trials, CMC studies and changes of biological products shall focus on safety risks, such as exogenous factors possibly introduced by raw material change and the possible effects of process changes on the removal of viruses/bacteria, and make quality attributes such as potency and purity comparable.
In principle, changes of biological products that have significant impacts on safety and efficacy during clinical trials should have been ended in confirmatory clinical trials with the manufacturing process of the drug substance basically stabilized, the composition and process established and the process scale and control representative, so that the confirmatory clinical trials may be more closely connected with commercial manufacturing in terms of scale, process, etc. CMC changes during the clinical period are encouraged to be supported by clinical trial safety and efficacy data.
Given the uncertainty in clinical development of biological products, CMC changes after the completion of confirmatory clinical trials are inevitable. Nevertheless, the major CMC changes that may affect the safety and efficacy of biological products are not generally recommended to be implemented during this period, unless sufficient supporting data are available.
3. Considerations for different types of biological products
The focus of CMC studies and changes during clinical trial varies for different types of biological products.
For innovative biological products, it is encouraged to design and develop based on the “Quality by Design (QbD)” framework. Due to the lack of prior knowledge for innovative biological products, more risk analysis and Design of Experiments (DOE) are required to gradually determine the relationship between critical process parameters (CPP) and critical quality attributes (CQA). In the early-stage clinical trials, safety related CPPs and in-process controls (IPCs) items and acceptable limits should generally be established, and necessary monitoring should be conducted for non-safety related CPPs and IPCs. With the advancement of CMC studies, the understanding of drugs and manufacturing processes should be deepened, and the scope for CPPs and IPCs should be gradually determined. During confirmatory clinical trials, it is encouraged to use processes for the proposed commercial manufacturing scale and to update and improve the specification.
For modified biological products, CMC studies and changes in each stage should be completed at appropriate time depending on the characteristics of the products, modification degree, the correlation of modification with safety and other information and taking into account of the development strategies during clinical trials.
For biological products to be applied based on data on marketed biological products (including biosimilars), thanks to the knowledge of same products, it is recommended to carry out comprehensive preclinical comparative study. During clinical trials, it is advisable to focus on local adjustment optimization and bridging study on the basis of commercial manufacturing scale to make preparation for marketing. When major CMC changes occur after the staged clinical trials and human data are waived, CMC studies should not only ensure subject safety but also support product efficacy evaluation.
4. Considerations for different categories of biological products
Traditional preventive vaccines (such as attenuated live vaccines, inactivated vaccines, etc) usually feature complicated material bases, difficult purification, difficult comprehensive characterization and different clinical subject groups from those for the therapeutic biological product, therefore, the requirements for CMC studies and changes during clinical trials should be different, but the basic concepts and principles shall keep consistent. In order to ensure the safety of subjects, in case of any change that have significant impacts, in addition to CMC comparability studies, it is recommended to carry out further non-clinical bridging studies, such as immuno bridging studies and necessary safety comparison studies. Although the correlation between evaluation indicators and protective efficacy of vaccines needs to be confirmed by confirmatory clinical studies, for the CMC changes in early-stage clinical trials, in addition to make the safety as the focus, it is appropriate to conduct CMC studies on efficacy assessment, for example, to compare and analyze the efficacy to induce humoral immunity, cellular immunity, etc. For a combined polyvalent vaccine, CMC studies of each active ingredient (antigen) should be gradually improved, and the safety and effectiveness of the vaccine product should be comprehensively assessed during clinical trials.
There are a wide range of genetically engineered recombinant protein products, including antibodies, fusion proteins, peptides and conjugated/modified proteins or peptides. Such biological products should be highly purified, and the primary structure, higher structure, post-translational modification, potency, product-related substances and impurities should be gradually and comprehensively confirmed using the existing advanced analytical methods. For products such as coupled/modified proteins or peptides, it is also necessary to study the quality attributes such as sites and proportion of modification/conjugation with reference to relevant technical guidelines. For any change occurred during clinical trials, corresponding non-clinical/clinical comparability bridge studies should be conducted when CMC comparability studies are not sufficient to exclude safety risks associated with such changes.
Human plasma used for manufacturing of blood products is a scarce resource. Due to the potential risk of viral contamination, viral safety control is the core of quality control of blood products. For the development of process of blood products, on the one hand, the impact on quality attributes such as virus safety and product potency should be focused; on the other hand, studies during clinical trials and the pre-marketing process validation scale and batches may be comprehensively considered taking into account of the improvement of the comprehensive utilization rate and the representative process.
5. Correlation between drug substance and drug product
The drug substance and the drug product are inseparable in CMC studies of a biological product; risks introduced by them are jointly embodied in samples used in clinical trials. The CMC studies and changes for drug substance and drug product should be improved gradually and respectively with the advancement of clinical trials. Meanwhile, because the drug substance and drug product may interact with each other, therefore, the CMC studies and changes during clinical trials should focus on the correlation between the R&D process of drug substance and that of drug product. If the change of drug substance has an effect on the drug product, the studies of drug substance and drug product should be carried out at the same time. If the change of drug substance has no effect on the drug product, the studies of drug product may be omitted.
(II) Risk assessment and comparability study of CMC changes
In order to ensure the safety of subjects and bridge the non-clinical/clinical study before the change, the effect of CMC changes on quality, safety and efficacy should be evaluated and comparability studies should be conducted for confirmation.
1. Risk assessment
During clinical trial, as the quality control system of biological products is gradually improved and data about the safety and efficacy are gradually obtained. Risks of even the same change may vary in different stages of clinical trials and for different types and different categories of biological products.
In early-stage clinical trials, because the cumulative knowledge of the process and product by the sponsor is limited and the safety of the drug in human has not been fully established, the risk assessment of the change may not be comprehensive. The possible impact of the CMC changes on the safety in subjects needs to be assessed taking into account of the results of non-clinical safety evaluation and early clinical studies. The results of clinical trials are the main basis for the evaluation of risk-benefit ratio for marketing of products. With the accumulation of knowledge, the risk evaluation system is gradually improved, and the risk evaluation of changes will be more mature. In the confirmatory clinical stage, in addition to the safety of subjects, the scientificity of clinical trial results shall also be taken into account. In the risk assessment of the changes after the completion of confirmatory clinical trials, it is necessary to take into account whether the quality management system of products is perfect. At the same time, for the risk assessment, all the changes shall be assessed item by item to make clear the reasons for changes and consider the potential risks caused by the changes.
So it is recommended to comprehensively analyze and evaluate potential risk factors and associated effects of biological product changes following the principle of case-by-case analysis. It is encouraged to conduct scientific risk assessment by referring to applicable guidelines such as ICH Q9.
2. Comparability study
The comparability study in the clinical trial is often affected by the R&D process, suitability of analytical method, and cognition level of process and product. For all CMC changes in clinical trials, appropriate comparability studies of the changes should be conducted with reference to ICH Q5E taking into account of the stage of occurrence, degree of impact of the changes and etc., to evaluate the impact of the changes on the quality, safety and efficacy of the drug.
The comparability studies in the early-stage clinical trials are generally not as comprehensive as those after marketing, and the quality is encouraged to be continuously improved without negative impacts on safety. With the accumulation of knowledge and process experience, the information used for comparability study will gradually increase. Generally speaking, for the CMC changes in later stage of clinical trials, comparability study shall be more comprehensive and systematic. If the change occurs after validation by confirmatory clinical trials, comprehensive comparability studies should be conducted in accordance with the ICH Q5E and requirements of post-market changes.
Comparability studies of CMC changes can comprehensively assess the impacts of changes in terms of IPCs (where applicable), release testing, expanded characterization studies and stability studies (forced degradation, accelerated and long-term stability).
Nonclinical studies and even bridging studies of clinical studies before changes and clinical studies after changes may be required if comparability results suggest that CMC changes may have negative impacts on the safety or efficacy data in clinical trials (e.g., changes of immunogenicity or occurrence of new impurities, etc.) or when the relationship between a specific quality attribute and safety and efficacy has not been established and there are differences in product quality attributes before and after the changes. For some changes that may exert significant impacts on clinical trials, e.g., use of a new master seed lot, changes of special excipients, prolongation of passage number of the viral seed for an attenuated live vaccine etc., if CMC analysis data alone may be inadequate to assess the impact of changes, nonclinical and/or clinical bridging studies should also be considered.
CMC changes during clinical trials usually do not occur independently. One change may be accompanied or followed by other related changes. It is recommended to make risk assessments depending on the practical situation. Overall, relevant comparability studies should be conducted based on the higher or even cumulative risks.
(III) Communication
Good communication helps the sponsor to make reasonable planning for the development process of the product, in order to control the risk caused by the changes and determine the application strategy based on the changes. In case of any change in the clinical trial, the sponsor may communicate and exchange with the drug regulatory authority on the CMC study of biological products and major technical issues related to the change (such as whether any further preclinical study is required if the change has an impact on product quality and safety), safety evaluation and risk management issues. In order to ensure the quality and efficiency of communication meeting, prior to the meeting, it is necessary to provide the overview of study during clinical trial and detailed study data in support of the change (as well as literature data), assessment of the possible impact on safety and efficacy and whether the existing study data support the clinical trial to be carried out, and clarify the topic of the meeting, the issues to be discussed and the preliminary solutions. The consensus formed through communication can be used as an important basis for subsequent R & D and evaluation.
For the detailed rules for communication, refer to relevant administrative provisions.
III. Phased requirements for CMC study (I) Drug substance (3.2.S) 1. Manufacture (S.2) 1.1. Manufacturer (S.2.1)
The name(s), address(es) and responsibilities of manufacturers (manufacture, testing), including contractors, and each proposed manufacturing site (manufacturing line) or facility involved in manufacture and testing, should be provided.
In case of any change to the manufacturer during clinical trial, it shall be fully evaluated whether such changes could cause risks in product quality and safety taking into consideration of the process, quality and stability of the said drug substance and conduct related studies.
1.2 Manufacturing processes and process controls (S.2.2)
It is necessary to specify the process flow (flow chart), describe each process step, including scale, culture medium and other raw materials and equipment for manufacturing and provide the process parameters and IPCs information. The storage and transportation conditions of drug substances shall be specified (if applicable). Information about safety assessment for control cells (if involved), adventitious agents in unprocessed harvests (unprocessed crude) should be updated based on the development progress (if applicable).
During early-stage clinical trials, information about process steps and intermediate control should be collected. Critical materials and reagents added in the process that may have an impact on safety should be monitored/controlled.
During confirmatory clinical trial, the manufacturing scale should be determined, the item and limit of process and process control should be improved based on the process development situation, and the initial acceptable limit of process and process control established in the early stage should be reviewed and revised (if applicable) to ensure that the manufacturing processes could be effectively controlled. Critical steps and IPCs should be specified.
In case of any changes to manufacturing process, scale and/or IPCs that affect drug safety and efficacy during clinical trials, the changes should be evaluated in accordance with ICH Q5E and the necessary comparability studies should be conducted. The scope of the comparability studies should be based on risk assessment of impact caused by the change and the clinical development stage. In principle, process changes during clinical trials should be more suitable for commercial manufacturing, and the process control capacity should be gradually improved.
1.3 Control of materials (S.2.3)
Cell bank/seed lot system:
Information on the generation, qualification and storage of the cell banks/seed lots should be updated as appropriate based on the actual development situation. The cell bank/seed lot should be fully qualified to ensure that the requirements of Chinese Pharmacopoeia and other relevant technical guidelines at home and abroad (such as ICH Q5A) are met. Attention shall be paid to the monoclonality of the seed lot/cell bank.
Generally, preliminary passage stability study data should be available in early-stage clinical trials. During confirmatory clinical trials, complete passage stability studies should be conducted, and reasonable in vitro passage limits/maximum population doubling level should be established. For vaccines, probiotics and other products, the cell substrate used/the passage number of bacteria/virus seeds should meet the requirements of corresponding general chapters and monographs in Chinese Pharmacopoeia.
Sufficient risk assessment and corresponding comparability studies shall be performed when a Working Cell Bank/Working Seed Lot (WCB/WSL) is established, a new WCB/WSL is established or in case of changes that affect the growth/passage characteristics of the WCB/WSL during clinical trials.
Raw materials for manufacturing:
Raw materials and used in the manufacture of the drug substance and consumables (including but not limited to starting materials, culture media, growth factors, enzymes, chromatographic column fillings, reagents, etc.) as well as manufacturing steps where they are used should be indicated, and necessary quality control should be performed. For materials of biological origin (including raw materials used in the cell bank/seed lot system preparation) and critical and complex raw material, the source, manufacture process (for self-made products), characterization (if applicable), specification and stability shall be specified, and the safety evaluation of exogenous agents (including TSE/BSE risk) shall be performed. For genotoxic raw materials/intermediates for manufacturing which could be introduced, the safety risk of the genotoxic substance should be fully assessed.
In case of any change to raw materials for manufacturing, the above information shall be updated accordingly, and the risks of exogenous agents and impurities that may be introduced shall be fully evaluated and necessary studies shall be conducted.
1.4 Control of critical steps and intermediates (S.2.4)
During early-stage clinical trials, operating ranges of process parameters should be preliminarily established, and related IPC parameters that may effect the safety of the products and acceptable limits should be established. For non-safety-related IPC parameters, necessary monitoring should be performed. Holding times and storage conditions for intermediates should be supported by primary physical, chemical, bioburden/sterility and other analysis data (if applicable). In confirmatory clinical trials, the IPC parameters and acceptable limits should be established gradually. CPP and IPC parameters and acceptance limits should be established prior to process validation. The acceptance criteria/limits for intermediates shall be improved to ensure the effective control of the quality of intermediates. The storage time and conditions of intermediates should be supported by study data.
1.5 Process validation and/or evaluation (S.2.5) 1.5.1 Viral removal/inactivation validation
The virus removal/inactivation validation should be performed by referring to Chinese Pharmacopoeia and relevant technical guidelines. If virus removal/inactivation platform process validation is used, full evaluation and confirmation shall be conducted by referring to relevant technical guidelines.
For genetically engineered recombinant protein products, the degree of virus removal/inactivation validation depends on the product development stage, and a comprehensive virus removal/inactivation process validation study and overall process virus safety risk assessment should be conducted before submission of the BLA.
For inactivated viral vaccines, studies in inactivators and inactivation process should be continued. The kinetic curve of virus inactivation should be established with at least multiple consecutive batches of samples to validate the inactivation efficiency. The inactivation process parameters shall be determined taking into account of the validation results, and shall meet the requirements of Chinese Pharmacopoeia before marketing.
In case of any change which has direct or indirect effect on virus removal/inactivation during clinical trial (such as change of material of nanofiltration membrane, change of type of inactivating agent), re-validation of virus removal/inactivation and safety risk assessment shall be conducted for the changed process.
1.5.2 Process validation/assessment
It is encouraged to collect data underlying and supporting process validation throughout the whole clinical trial period to support completion of process performance qualification (PPQ) before submission of the BLA. Prior to the submission of the BLA, in principle process validation should be conducted on at last 3 consecutive batches under commercial-scale conditions to ensure the robustness of the process and consistency of product quality.
Usually, for upstream culture process validation, consideration should be given to cell morphology, growth characteristics, density, viability, cell metabolism level, expression level of target products, cell stability, etc.; for bacterial/viral vaccines, consideration should also be given to virus titer or cell/bacterial seed activity (if applicable), target antigen content and purity (if applicable), etc. For polysaccharide protein conjugated vaccines, consideration should also be given to the derivation rate, derivation/binding kinetics, and specific carrier protein monomer or polymer forms. For purification process validation, consideration should be given to purity, product-related impurities and process-related impurity removal capacity. For chemical-coupling modified biological products, degree of modification, content of free small molecules, proportion of unconjugated protein and yield shall also be confirmed. Validation of cleaning/storage/regeneration and cycle life of ultrafiltration membrane pack/chromatography media, compatibility assessment and study of equipment/container in direct contact with drug substance, intermediate storage stability validation and transportation validation (if applicable) shall be carried out.
1.5.3 Process development
The development process of manufacturing process shall be described, with the reasons for change specified and the changes summarized. The batch numbers and use of drug substance using the representative processes during development should be summarized. For changes at different stages of development, corresponding comparability studies shall be conducted to determine the impact of the changes.
2. Characterization (S.3) 2.1 Structure and physico-chemical properties (S.3.1)
The characteristics study (including physical and chemical properties, potency, immunochemical characteristics, purity and impurities, etc.) shall be continuously improved throughout the whole clinical trial period; the basis for selection of the characteristics analysis method used and its suitability shall be described.
During early-stage clinical trials, knowledge about structures and physico-chemical properties should be continually accumulated; during confirmatory clinical trials, full characterization should be performed using advanced technical means and methods, including primary and higher-order structures, purity and potency studies, etc., to provide a basis for understanding the relationship between product structures and functions, identifying CQA of the product and developing analysis and control strategies.
For vaccines, during clinical trials, taking into account of the types and characteristics of vaccines, the expanded quality studies shall be continuously promoted to further confirm the protective antigen component, content and conformation of vaccines and accumulate the correlation between the quality of protective antigens and clinical immune effect. For expanded quality studies, the followings shall be included (but are not limited to): specific identification of antigens, physico-chemical properties, structural/sequence variation (if relevant), purity and impurity analysis, infectivity (if relevant), potency (such as antigenicity and immunogenicity) related to vaccination effect. For adjuvanted vaccines or combined polyvalent vaccines, comprehensive studies on interactions between adjuvants and antigens and interactions among antigens should be continuously conducted during clinical trials.
For blood products, consideration may be given to the extended quality studies on the product function related components (e.g., vWF in coagulation factor VIII), product activation status, components that may affect potency (e.g., profibrinolysin in fibrinogen) taking into account of the product type, drug product type and drug development stage.
The product shall be subject to full characterization and comparative analysis in case of any major process change during the clinical trials.
2.2 Impurities (S.3.2)
During clinical trials, the analytical study on product-related impurities (such as precursors, cleaved forms, degradation products, aggregates, etc.) and process-related impurities (such as host cell proteins, host cell DNA, media residues, etc.) should be continuously improved. Quantitative information (including that at maximum clinical dose) on impurities should be specified. With adequate justification, a qualitative study of some impurities or only the clearance assessment for process-related impurities (e.g. antifoam, etc.) can be performed. For new impurities (if applicable), qualitative and quantitative studies should be conducted for risk assessment, and acceptance limits should be established based on comprehensive consideration.
Prior to the submission of the BLA, the impurity in the biological product, degradation mechanism of product and corresponding changes of product-related impurities during storage should be clarified, and risk control strategies should be developed to ensure product safety.
3. Quality control (S.4) 3.1 Specification and justification (S.4.1 & S.4.5)
Specification of a drug substance should include CQAs, e.g., content, identity, purity and impurities, potency, physico-chemical properties, sterility/microbial limits, endotoxins, etc. During clinical trials, process validation/evaluation data are incomplete, the quality control should not be limited to the tests included in the specification.
In early-stage clinical trials, the acceptance criteria for content, identity, purity (principle peak) in the specification of the drug substance should be widened and not be defined as “report results”; limits for impurities and microbiological safety should be reasonably set; for quality attributes for which sufficient data are required and product characterization studies should be taken into account to establish reasonable limits (e.g., glycosylation, charge heterogeneity), the “report result” is acceptable.
During the period from confirmatory clinical trials to submission of the BLA, criteria or limits should be established for tests with acceptance criteria defined as “report results” in the specification. The specification should be based on relevant development data, platform technology, manufacturing data on batches in nonclinical and clinical studies, quality properties and stability study data, meanwhile, take into account testing performance of test methods.
For biological products to be applied based on data on marketed biological products (including biosimilars), the general technical requirements in Chinese Pharmacopoeia shall be met. In principle, the specifications shall not be lower than those of the marketed similar products.
In case of specification changes during clinical trials, the previous specification should be reviewed and adjusted based on the clinical development stage, and the batch release and stability (if applicable) test results of representative samples shall be used for supports.
3.2 Analytical methods and validation (S.4.2 & S.4.3)
In the early-stage clinical trials, the suitability of test methods should be preliminarily confirmed, and corresponding methodology studies shall be carried out taking into account of the importance of quality attributes and R&D stage. If it is involved, sensitive, specific and different test methods should be established for the identification and safety analysis of newly added impurities or degradation products, and reasonable control strategies should be considered based on the safety analysis results. It is encouraged to establish a potency analysis method that can reflect the mechanism of action of the drug as early as possible.
Normally, during confirmatory clinical trials and prior to process performance qualification, method qualification or comprehensive method validation should be conducted according to the requirements of pharmacopoeias and relevant technical guidelines.
If analytical methods have been optimized or improved during clinical trials, method bridging studies and assessment (if applicable) should be performed; in principle, post-change analytical methods should be not inferior in testing performance to pre-change analytical methods.
3.3 Batch analysis (S.4.4)
Information about released batches should be summarized in a tabular format, including batch number, batch size, manufacturing site, manufacturing date, specification and test results, process version as well as batch use; and batch analysis data of critical batches in nonclinical and/or clinical trials in support of BLA.
4. Standard substance (S.5)
The selection and establishment of standard substances is one of the key factors to measure the consistency of different batches of drugs during clinical trials, as well as the comparability between drugs to be marketed and drugs used in clinical trials. State-of-the-art analytical methods should be used to fully characterize the standard substances and calibrate the potency. Although it is ideal to use the same standard substance for biological test and physical and chemical properties study, sometimes based on the actual situation, different standard substances shall be used for the study of physical and chemical characteristics, potency and related substances, respectively. It is encouraged to establish in-house primary standard substances as early as possible. The working standard substances shall be calibrated with primary Standard substances.
In case that international or national standard substances are available, they can be used as the primary standard substances, and used to calibrate the in-house standard substances. It should be noted that the use of some standard substances may be limited to some specific test methods. It is required to establish primary standard substances for drug-related substances, drug-related impurities and process-related impurities in order to calibrate subsequent working standard substances (if applicable).
In case of no international or national standard substances, in-house primary standard substances shall be established. For in-house standard substances made using different process during clinical trials, comprehensive characterization and stability testing should be conducted to ensure that the standard substances in different stages are traceable. It is usually recommended primary standard substances be established with batches of the representative process used in confirmatory clinical trials and process validation batches, and after complete characterization of the primary standard substances, the working standard substance can be calibrated with such primary standard substances.
5. Container closure system (S.6)
The container closure system used for transportation and/or storage of drug substances during clinical trials shall be specified, and it shall be proved that such container closure system will exert no adverse impacts on the quality of drug substance. The compatibility and closure-integrity studies of storage containers for drug substances shall be conducted during confirmatory clinical trials (if applicable).
In case of any changes to the container closure system of drug substances during the clinical trial, the impact on quality and stability of such drug substances should be evaluated, and compatibility and closure-integrity studies should be carried out (if applicable).
6. Stability (S.7)
The stability data of relevant drug substance should be summarized, specifying the batches, date of manufacture, process version, use, storage conditions, time-points, specification and test results.
A small-scale container closure system with the same composition as the actual packaging material can be used for drug substance stability study. Analytical procedures with suitable stability indicating properties shall be used to maximize the possibility of detection of the changes in the purity, impurity and potency of the drug substances. Trend analysis and evaluation of stability sensitive indicators are required. In case of sufficient evidences, CQAs that do not change during storage may not be included in stability testing.
During early-stage clinical trials, stability study data and stability profiles of the drug substance shall be accumulated gradually. Stability study data should be supportive of the conduct of subsequent clinical trials.
During the period from the confirmatory clinical trials to submission of the BLA, the long-term stability study of the drug substances should be continued with reference to the Chinese Pharmacopoeia, ICH and other relevant technical guidelines, and consummate stressed tests (e.g., extreme pH, light, vibration, freezing-thawing, high temperature, oxidation, etc.) and accelerated tests to confirm the potential degradation pathways of drug substance and comprehensively understand the stability characteristics of drug substance, so as to provide the basis for the establishment of storage period.
(II) Drug product (3.2.P) 1. Product development and manufacturing (P.2 & P.3) 1.1 Composition and batch formula (P.2.1 & P.3.2)
The dosage form, composition (batch formula), source information on all components, function and specification of the product used during the clinical trials shall be specified. If any new excipients are used, there should be sufficient justification and safety data for supports. The batch size information on representative batches used in clinical trials shall be clarified. If applicable, it is necessary to specify the source of accompanying diluent, formulation and the specification of the diluent excipients (if any) should be specified.
Prior to confirmatory clinical trials, the composition and dosage form of the drug product should be defined. If it involves the composition of some drug products that are delivered through the device, it shall be generally similar to that of the drug to be marketed. If applicable, the new drug delivery device for use in confirmatory clinical trials should be subject to safety validation and assessment and kept consistent with that to be marketed.
If the changes in composition and drug delivery device may affect drug quality, stability, safety and clinical use, the reasons for the changes in clinical trial stage shall be explained with corresponding study data used for supports.
1.2. Manufacturer (P.3.1)
The name(s) and address(es) and responsibilities of manufacturers (manufacture, testing) of drugs used in clinical trials, including contractors, and each proposed manufacturing site or facility involved in manufacture and testing, should be provided.
In case of any change to the manufacturer during clinical trial, it shall be fully evaluated whether such changes could cause risks in product quality and safety taking into consideration of the process, quality and stability.
1.3 Manufacturing processes and process controls (P.3.3)
It is necessary to specify the process flow (flow chart), describe each process step, provide process parameters and IPCs information, and gradually improve IPCs test items and acceptable limits. Regarding the preparation of final bulks (if applicable), the numerical values for the quantities of active ingredients or active units added should be specified. For vaccines to which addition of adjuvants is needed, the studies of necessity and doses of added adjuvants should be continually conducted. For sterilization by filtration, attention should be paid to the maximum acceptable bioburden prior to the filtration.
Necessary comparability studies shall be conducted in accordance with the ICH Q5E if there are changes in the manufacturing processes and IPCs (e.g., lyophilization, adsorption, lipidosome encapsulation/packaging) of drug product during the clinical trials.
1.4 Control of critical steps and intermediates (P.3.4)
In the clinical trials, the process parameters and limits of critical steps should be determined gradually.
During early-stage clinical trials, the control strategy should focus on safety-related IPCs with acceptable limits for safety-related IPCs established; other IPCs should be monitored. In case of storage of intermediates, there shall be sufficient reason. The storage time and conditions shall be supported by data.
In the confirmatory clinical trials, IPC items and acceptance criteria/limits in the process flow are established and CPPs shall be gradually identified and qualified. The manufacturing batch size and scale should be defined.
1.5 Process validation and/or evaluation (P.3.5)
Prior to the submission of the BLA, the process validation shall be completed at the manufacturing scale to be marketed, so as to confirm and assess the robustness of process and between-batch consistency of product quality. The state of validation of aseptic filling process and lyophilization process should be described, if applicable.
For sterile drugs or non-sterile drugs of multi-dose container closure system, if the changes in composition, pH and container closure system may have an effect on the test method for antimicrobial efficacy or antimicrobial efficacy, the antimicrobial efficacy test method shall be re-validated/verified, and the antimicrobial efficacy shall be monitored at key time points of long-term stability study. For products with manufacturing processes including terminal viral inactivation (e.g., dry heat inactivation), if a process change may affect terminal viral inactivation, re-validation shall be conducted.
2. Control of excipients (P.4 & A.3)
Generally, the excipients listed in the pharmacopoeia shall be used. The used excipients shall meet the needs for the drug product, and the in-house specification should be established. For the excipients of human or animal origin, the information on the safety assessment of exogenous factors and the TSE/BSE risk-free statement should be provided. For excipients and adjuvants used for drugs for the first time or for new administration route, it is recommended to conduct studies by referring to relevant technical guidelines at home and abroad and to continuously improve such studies during clinical trials.
3. Quality control (P.5)
3.1 Specification and justification (P.5.1 & P.5.6)
The basic principles for quality studies and controls of the drug product are the same as those for the drug substances. Generally, the specification of drug products should include at least content, identity, purity and potency tests, and the control of composed critical excipients, special excipients, adjuvants and other functional components added should also be considered. For sterile drugs, sterility and bacterial endotoxin testing is required. If involved, impurities not covered in the drug substance testing (e.g., introduced during the manufacturing and/or storage of the drug product) shall be qualified and quantified.
During early-stage clinical trials, preliminary acceptance criteria may be established based on limited development and batches used in nonclinical and clinical trials. For some testing items, "report results” could be acceptable. In principle, levels of impurities in samples used in clinical trials should not exceed those supported by animal safety studies, previous clinical trials or prior knowledge (such as platform technology), and upper limits should be set for impurities if necessary.
In confirmatory clinical trials, the factors such as correlation between specification and manufacturing process, stability of drug substance and drug product, compatibility, historical batch data used in pre-clinical and clinical studies and analytical method characteristics shall be considered to improve and establish the specification for the drug products. For the drug product containing multiple active ingredients, the specification shall be established based on the content, purity and potency of each active ingredient taking into consideration of the clinical dosage to ensure that the test method used can accurately distinguish one component from another. For a multi-dose drug product, the accuracy of dosage and in-use microbial limits should be ensured (if applicable). For sustained-release drug products, controlled-release products, enteric-coated products and transdermal patches, drug dissolution/release studies should be carried out. For vaccines, test parameters that can comprehensively characterize humoral or cellular immunity effects should be included depending on characteristics of the vaccines, if applicable. For combined vaccines, interactions among components as well as the impact of adjuvants on the active ingredients and testing should be continually studied.
For biological products to be applied based on data on marketed biological products (including biosimilars), the general technical requirements in Chinese Pharmacopoeia shall be met. In principle, the specifications shall not be lower than those of the marketed similar products.
In case of specification changes during clinical trials, the previous specification should be reviewed and adjusted based on the clinical development stage, and the batch release and stability (if applicable) test results of representative samples shall be used for supports. In case that heterogeneity of the target product is inconsistent with the product used for clinical trials due to process changes or drug degradation, the impact of these changes should be evaluated and necessary studies shall be conducted.
3.2 Analytical methods and validation (P.5.2 & P.5.3)
See Section Drug substance for reference.
3.3 Batch analyses (P.5.4)
See Section Drug substance for reference.
4. Standard substance (P.6)
See Section Drug substance for reference.
5. Container closure system (P.2.4 & P.7)
It is necessary to make clear the source and standard of packaging materials, provide the qualified information on packaging materials and describe the relevant filing and registration information (if any). The packaging materials should be those listed in the pharmacopoeia or national standards for pharmaceutical packaging materials and shall comply with the requirements in applicable technical guidelines in China and other countries. If the packaging material is non-standard drug delivery device such as inhalation aerosol device and disposable injection device or drug delivery device made of novel material, it is necessary to make clear the basis for use and the proposed quality standard shall be met.
During early-stage clinical trials, preliminary closure-integrity and compatibility studies should be generally conducted to prove the container closure system will not have negative influence on the product quality.
During the confirmatory clinical trials, comprehensive closure-integrity and compatibility studies should be carried out by referring to relevant guidances and guidelines. The studies of drug delivery device shall be done under simulated actual use conditions before BLA submission to prove the repeatability and accuracy of administered dose. In principle, the container closure system in the confirmatory clinical trials shall be consistent with that to be marketed.
In case of any changes to the container closure system of drug products during the clinical trial, the impact on quality and stability should be evaluated, and compatibility and closure-integrity studies should be carried out.
6. Stability (P.8)
For the basic considerations and phased requirements for the stability study of drug products, see Section Drug substance for reference.
Drug product stability studies shall consider the general aspects of stability studies of the drug substances. In the early-stage clinical trials, stability studies should be able to support the development of staged clinical trials. In the confirmatory clinical trials, the quality and packaging materials of the drug product batches in the stability study should be consistent with those of the products to be marketed, and comprehensive stability testing should be conducted by referring to the Chinese Pharmacopoeia and ICH guidelines to provide supportive data for the establishment of shelf life and drug usage. For the stability studies of drug products, different placement directions (such as upright, inverted and horizontal placement) should usually be used. For separately packaged diluent/adjuvant, in addition to the stability investigation of each packaging component, the stability after dilution/mixing covering the shelf life shall be investigated. For drug products or multi-dose products that are required to be used after reconstitution, dilution, mixing and holding, in-use stability studies shall be conducted. In-use stability of samples near the end of shelf life or representative samples near the end of shelf life is encouraged for investigation.
IV. Evaluation of CMC changes and changes that may increase safety risks
According to Provisions for Drug Registration, in case of any CMC changes or new findings of biological products during drug clinical trials, the impact on the safety of subjects shall be fully evaluated. For the changes, if the sponsor assesses that subject safety is increased, a supplementary application shall be submitted in accordance with the relevant requirements of the Provisions for Drug Registration; if the sponsor assesses that subject safety is not affected, the changes may be directly implemented and reported in the Development Safety Update Report. For CMC changes to biological products after the completion of confirmatory clinical trials, supplemental applications may be submitted before or during BLA submission.
During clinical trials, CMC changes of biological products that may increase safety risks are as follows. It is recommended that the sponsor focus on the examples listed. However, it is still necessary to carry out evaluation and study and determine the application path in accordance with the above risk grading principles. It is recommended to communicate in case that the judgment results are different.
Supplemental applications may include new obtained CMC safety date, updates to previously submitted CMC safety information and dossiers, dossier about CMC studies that ensure follow-up clinical trials and other safety related study data. Safety update report should document in detail all information about CMC studies and changes during clinical trials, including supportive or confirmatory study data, CMC study overview as well as replies to comments from the drug review agency (if any).
CMC changes of biological products with potential safety risks identified but not actually implemented in clinical subjects usually do not affect the completed or ongoing clinical trials; however, if CMC safety problems or other risks are found in the clinical trials, the clinical trials should be immediately suspended or terminated and the potential serious safety risk information should be reported to the drug review agency in accordance with the Standards and Procedures for Rapid Reporting of Safety Data during Drug Clinical Trials; and the clinical trials can be continued only after the safety problems are solved or eliminated through supplementary studies.
For some changes involving the changes in the material basis of biological products, it is necessary to submit a new clinical trial application in accordance with the Provisions for Drug Registration and the Requirements for Registration Classification and Application Dossiers of Biological Products, such as the vaccine using new bacterial strains and new adjuvants.
CMC changes to biological product that may generally increase safety risk are as follows:
1. Drug substance 1.1 Control of materials
1) New master cell bank/master seed lot.
2) Substantial changes to critical raw materials.
1.2 Manufacturing processes
3) Substantial change of manufacturing site.
4) Changes to the fermentation process that adversely affect safety.
5) Changes to purification process flow or purification process changes that result in the generation of new impurities/new product-related substances.
6) Changes in manufacture processes that directly and indirectly affect virus inactivation/removal.
7) Changes (widening, deletion) of the scope of IPCs related to safety.
1.3 Quality control
8) Major changes to specification that affect the safety of the drug substance (except for specification that could increase the safety).
1.4 Packaging materials and stability
9) Changes in storage conditions that may exert adverse effect on the product quality.
2. Drug Product
2.1 Composition
1) Changes in composition or dosage form (including changes in active ingredient concentration and composed excipients, water for injection changed into powder for injection, injection in vials changed into prefilled injection).
2) Changes to excipients that may exert adverse effect on the safety of the drug product.
3) Changes in adjuvants.
2.2 Manufacturing processes
4) Substantial change of manufacturing sites (except for changes of manufacturing sites for secondary packaging).
5) Changes in manufacture processes that directly and indirectly affect virus inactivation/removal.
6) Changes in manufacture processes of drug products that affect sterility and impurity clearance.
7) Changes (widening, deletion) of the scope of IPCs related to safety.
2.3 Quality control
8) Major changes to specification that affect the safety of the drug product (except for the addition of safety control).
2.4 Packaging materials and stability
9. Container closure system in direct contact with drug product (such as materials).
10) Change in storage conditions that may adversely affect product quality.
3. Any new CMC information that may adversely affect the safety (e.g. identification of new impurities, increased TSE risk, etc.).
4. Other significant changes affecting safety or CMC changes due to safety reasons.
V. Reference Guidances
1、《新药I期临床试验申请技术指南》(国家药品监督管理局2018年第16号通告附件),2018.1。
2、《创新药(化学药)Ⅲ期临床试验药学研究信息指南》(国家药品监督管理局 2018 年第48号通告附件),2018.3。
3、《创新药(化学药)临床试验期间药学变更技术指导原则(试行)》(国家药监局药审中心2021年第22号通告附件),2021.3。
4、《新型冠状病毒预防用疫苗研发技术指导原则(试行)》(国家药监局药审中心2020年第21号通告附件),2020.8。
5、《新型冠状病毒中和抗体类药物申报临床药学研究与技术资料要求指导原则(试行)》(国家药监局药审中心通告附件),2020.9。
6、《血液制品去除/灭活病毒技术方法及验证指导原则》(国药监注[2002]160号)
7、《生物制品稳定性研究技术指导原则(试行)》(原国家食品药品监督管理总局 2015年第10号通告附件),2015.4。
8、《化学药品注射剂包装系统密封性研究技术指南(试行)》(国家药监局药审中心2020年第33号通告附件),2020.10。
9、《化学药品注射剂生产所用的塑料组件系统相容性研究技术指南(试行)》(国家药监局药审中心2020年第33号通告附件),2020.10。
10、《化学药品注射剂与药用玻璃包装容器相容性研究技术指导原则(试行)》(原国家食品药品监督管理总局2015年第40号通告附件),2015.7。
11、FDA Guidance for Industry: IND Meetings for Human Drugs and Biologics; Chemistry, Manufacturing and Controls Information , 2001.5。
12、Guidance for Industry on INDs for Phase 2 and Phase 3 Studies Chemistry, Manufacturing, and Controls Information, FDA, 2003.5。
13、 Guideline on the requirements for quality documentation concerning biological investigational medicinal products in clinical trials, EMA, 2022.2。
14、 ICH Q5C: Stability Testing Of Biotechnological/Biological Products, 1995.11。
15、ICH Q5D:Derivation And Characterisation Of Cell Substrates Used For Production Of Biotechnological/Biological Products ,1997.7。
16、ICH Q5E:Comparability of Biotechnological/Biological Products Subject to Change in Their Manufacturing Process, 2004.11。
17、ICH Q6B:Specifications: Test Procedures And Acceptance Criteria For Biotechnological/Biological Products,1999.5。
18、ICH Q8(R2): Pharmaceutical Development,2009.8。
19、ICH Q12: Techinical And Regulatory Considerations For Pharmaceutical Product Lifecycle Management,2019.11。
VI. Glossary
Confirmatory clinical trial: refers to a clinical trial during which core efficacy data are available to support marketing.
Related changes: refer to other changes that are accompanied by or triggered by one change.
Comparability study in clinical trials: activities including trial design (study samples, analytical methods, and pre-determined comparability acceptance criteria, etc.), study conduct, and data evaluation to assess whether products are comparable before and after the change.
Prior knowledge: refers to the existing knowledge, including internal knowledge (such as development and manufacturing experience), external knowledge (such as scientific publications, including the data of supplier, literature and peer review publications) or the established scientific principles (such as chemical, physical and engineering principles).
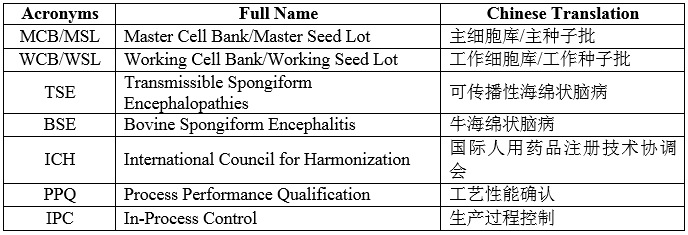
VII. List of Acronyms


 Law & Regulations
Law & Regulations
 Position :
Position :